PuriZorb-PG4
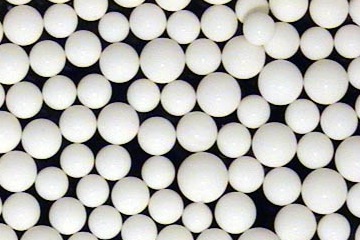

PuriZorb-PG4 (Product Information Sheet)
PuriZorb-PG4 is a crosslinked polystyrene-divinylbenzene resin with a macroreticular structure, designed for adsorption of hydrophobic solutes and detergents from aqueous environments.
- Standard format: 2L sterile polycarbonate bottle (customizable upon request)
- Buffer: PBS, endotoxin-free (customizable upon request)
- Resin matrix: Polystyrene-divinylbenzene, macroreticular bio-beads
- Mean pore diameter: ~40 Å
- Surface area: ~750 m²/g
- Particle size: 20–60 mesh
- Sterility: Shipped sterile and endotoxin-free
- Stability: Chemically inert, autoclavable (121 °C)
- Reusability: Resin can be regenerated with mild solvents and/or autoclaving
- Tested for residual DVB and TCO (total chromatographable organics)
Performance Highlights:
- Effective adsorption of non-ionic detergents such as Triton X-100 and Tween (polysorbate)
- Effective adsorption of ionic detergents such as CTAB and Deoxycholate.
- Effective removal of Sarkosyl to concentrations below its CMC, enabling protein refolding.
- Partial removal of zwitterionic detergents (CHAPS).
- Minimal protein binding, preserving yields.
- Successfully applied in subunit vaccine production (e.g. Influenza vaccine).
Innovative solutions for biotechnological purification processes
Discover our expertise in cGMP manufacturing and advanced life sciences technologies, where we combine quality and innovation.

Vaccine Applications
Efficient removal of detergents such as Triton X-100, SDS, CTAB, and Sarkosyl after viral inactivation or protein solubilization, ensuring clean bulk material for downstream vaccine processing. Well known for purification of antigens from Influenza Strains (Hemagglutinine (HA) and Neuraminidase (NA))
Protein Purification
Facilitates the refolding and stabilization of membrane proteins or inclusion body-derived proteins by completely eliminating residual detergents — a strategy successfully applied in COVID-19 subunit vaccine development
Liposome & Virosome Reconstitution
Enables the removal of detergents used to solubilize phospholipids, allowing functional vesicle formation. Supports detergent-mediated reconstitution of lipid-based carriers, delivering consistent, high-quality liposomes and virosomes.
Endotoxins & Trace Impurity Clearance
Adsorbs hydrophobic contaminants such as phenols, pesticides, and small organic molecules, improving the purity of pharmaceutical, biological, or food-grade preparations.
Polysaccharide Purification (e.g., Hib)
Resin-assisted clarification of polysaccharide preparations, such as Haemophilus influenzae type b, by removing hydrophobic by-products and process reagents, resulting in high-purity vaccine components.
Innovations in cGMP manufacturing processes for life sciences
Discover our advanced technologies and expertise.

Biotechnological Purification
Optimizing purification processes for maximum effectiveness.
Process development
Development of efficient and scalable production processes.
Consultancy
Customized advice for cGMP compliance and innovation.
Quality control
Careful monitoring to ensure the highest product standards.
Get in Touch with Purivise Today
Partner with us to enhance your biopharmaceutical purification workflows. Our team is ready to provide expert guidance, tailored solutions, and innovative products like PuriZorb-PG4 to support your success. Connect with us today and discover how Purivise can empower your next breakthrough.