Applications

Advanced biotechnological purification technologies
Purivise stands for innovation in life sciences, with a focus on cGMP production and biotechnological purification. Our core values of quality, reliability, and sustainability ensure our long-term success and growth.
Smart Solutions for Biotechnology and Purification
Explore our portfolio of technologies and potential applications, designed to elevate biopharmaceutical manufacturing.
Vaccine Production
During the manufacture of vaccine antigens (e.g. Influenza virus, Varicella-Zostervirus ), viral membranes are first solubilised with mild detergents such as Triton™ X-100, Sodium Deoxycholate or CTAB to release surface glycoproteins. Subsequent adsorption on PuriZorb-PG4 efficiently removes the detergents while maintaining the native conformation of e.g. hemagglutinin (HA), neuraminidase (NA) and glyocprotein (gE). This controlled step yields purified antigens with low surfactant residues, supporting the production of safe, stable and immunologically active Influenza or VZV vaccine components.
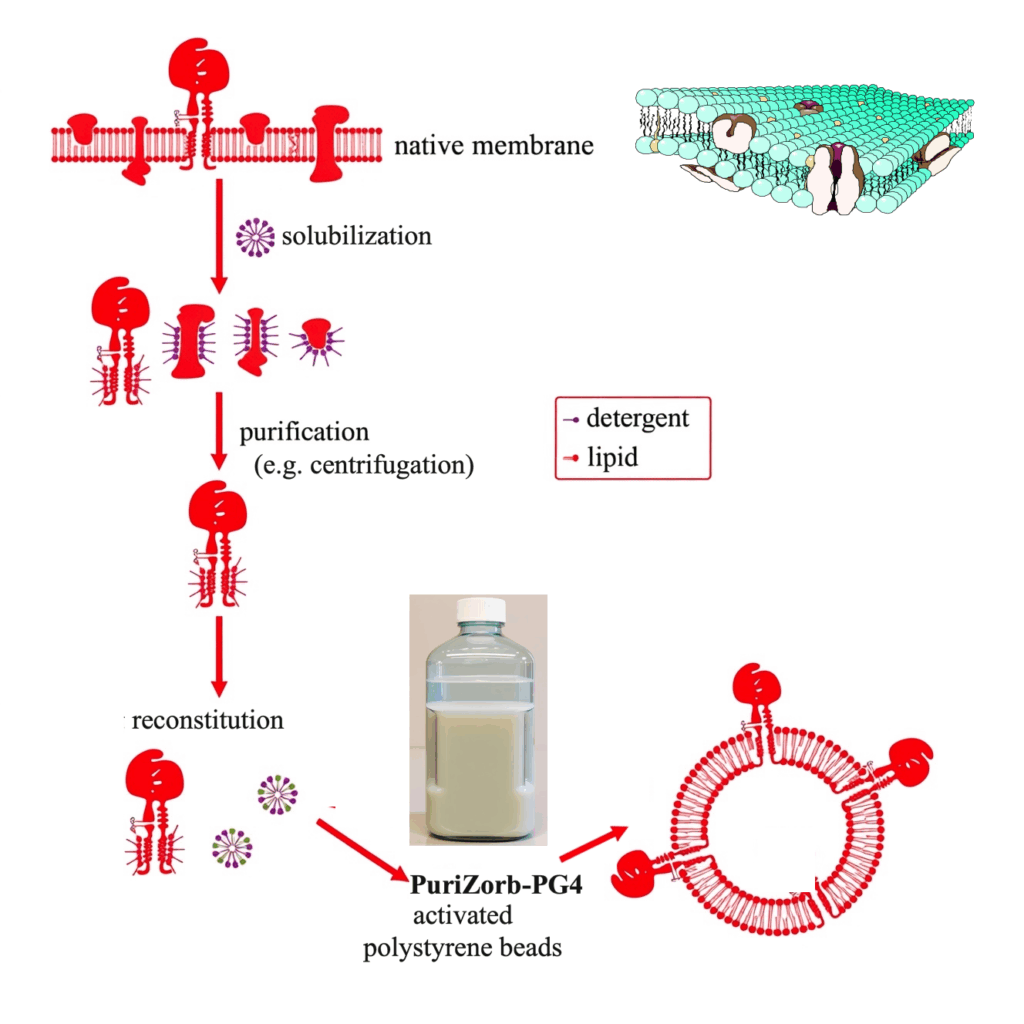
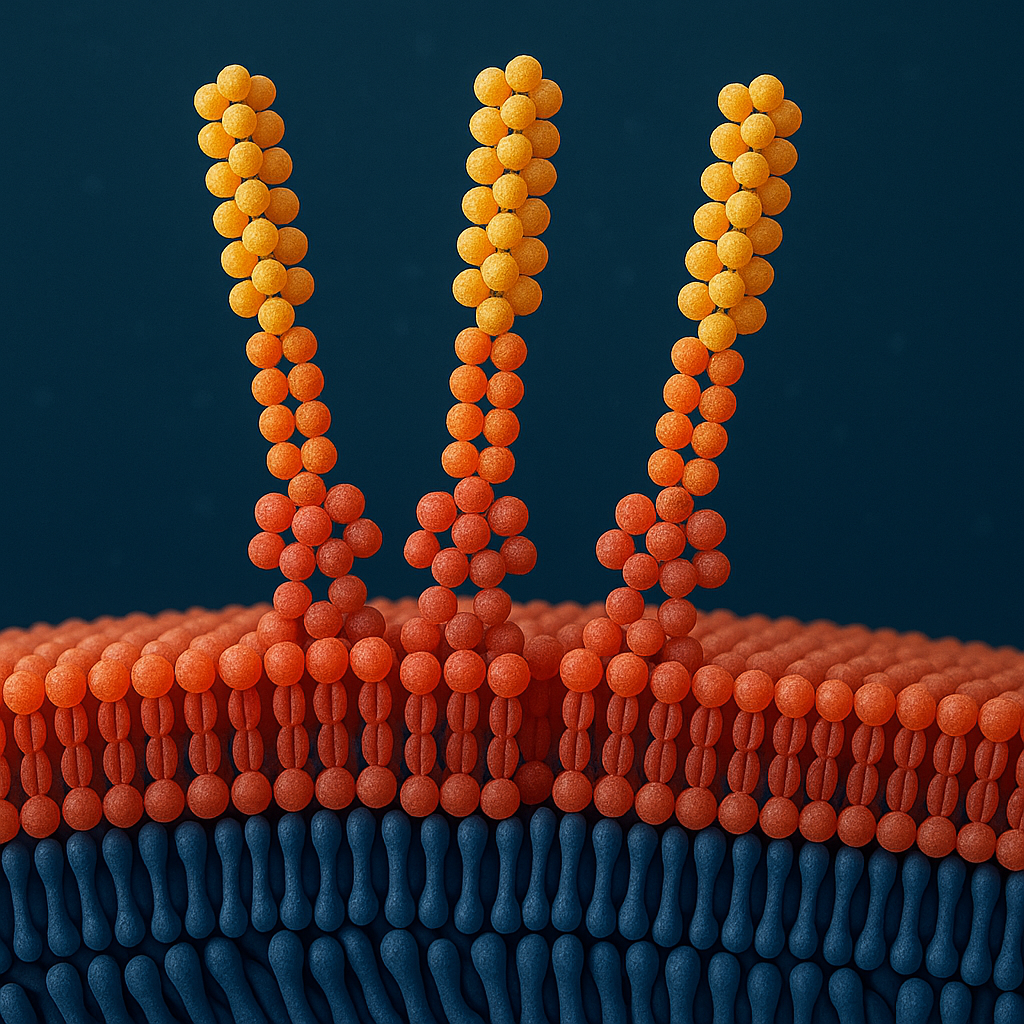
Membrane protein purification
Membrane proteins are often first extracted from cellular membranes using detergents to disrupt the lipid environment and release the target proteins into solution. After solubilisation, PuriZorb-PG4 is applied to selectively remove the detergent. This step promotes proper refolding or reinsertion of the membrane protein into a stabilising lipid or mild micellar environment, helping to preserve its native structure and activity. By reducing residual surfactant, the process improves purity, minimises interference in downstream assays, and yields membrane proteins suitable for biochemical characterisation, structural studies, or therapeutic applications.
Liposome and Virosome formation
During the preparation of liposomes or virosomes, PuriZorb- PG4 is often used to remove detergents after lipid solubilisation. As lipids self-assemble into bilayers, the resin selectively adsorbs the detergent, gradually shifting the equilibrium toward closed vesicles. This gentle process preserves the structural integrity of embedded proteins or antigens and enables the formation of uniform, stable particles. By efficiently clearing residual surfactant, PuriZorb-PG4 helps produce high-quality liposomes and virosomes suitable for pharmaceutical, vaccine, or diagnostic applications.
Endotoxin removal
Endotoxins can be efficiently removed from pharmaceutical or medical solutions and suspensions by combining a mild detergent wash with adsorption on Purizorb-PG4 resin. The detergent disrupts lipopolysaccharide aggregates and improves accessibility of their hydrophobic domains. Purizorb-PG4 then captures the released endotoxins through its porous, hydrophobic matrix while allowing proteins or other actives to remain in solution. After thorough washing, the cleaned product is recovered by simple filtration, providing a low-endotoxin preparation suitable for sensitive therapeutic applications.
Macrolide purification
Macrolide antibiotics (e.g., erythromycin, clarithromycin, spiramycin) can be efficiently purified from fermentation broths using hydrophobic adsorption on PuriZorb-PG4 resin. At neutral to slightly basic pH, macrolides bind strongly to the non-ionic polystyrene–divinylbenzene matrix, while sugars, salts and other polar by-products are washed away. The adsorbed product is then eluted with 50–80 % methanol or isopropanol, often with a small amount of ammonium hydroxide to maintain stability. This method offers high recovery, effective removal of impurities, and concentration of macrolides in a single step.
Get in Touch with us Today
Partner with us to enhance your biopharmaceutical purification workflows. Our team is ready to provide expert guidance, tailored solutions, and innovative products like PuriZorb-PG4 to support your success. Connect with us today and discover how Purivise can empower your next breakthrough.